Safety Concerns with Nanotechnology in Medicine
Objective:
To explore the safety concerns associated with nanotechnology in medicine, focusing on its potential risks and strategies for mitigating these concerns.
Introduction to Safety Concerns:
As nanotechnology increasingly finds applications in medicine, especially in neurosurgery, the potential for groundbreaking advancements in treatment and diagnostics is immense. However, the small size of nanomaterials introduces a unique set of safety concerns. These concerns primarily relate to the interactions between nanoparticles and human cells, tissues, and organs, which may be difficult to predict given the novel nature of these materials. Understanding these risks and developing strategies to mitigate them is crucial for the safe implementation of nanotechnology in clinical practice.
Key Safety Concerns in Nanotechnology:
- Toxicity of Nanomaterials:
- Nanoparticles can behave very differently from their bulk counterparts. Their small size and large surface area can increase their reactivity and potentially cause harmful interactions with human cells. This could lead to toxicity, inflammatory responses, or other adverse effects.
- Example: Some nanomaterials, like certain metals (e.g., silver nanoparticles), can generate reactive oxygen species (ROS) that may damage cells, tissues, or DNA.
- Long-Term Health Effects:
- The long-term effects of exposure to nanomaterials are still not fully understood. Since nanoparticles can easily enter cells and tissues, there is concern that they might accumulate over time in vital organs such as the liver, kidneys, or brain, potentially leading to chronic toxicity or even carcinogenesis.
- Example: In animal studies, the accumulation of certain nanoparticles in the liver has been observed, raising concerns about potential long-term organ damage.
- Environmental Impact:
- Aside from direct health concerns, there are questions about the environmental impact of nanomaterials. The production, use, and disposal of nanoparticles could lead to contamination, with nanoparticles potentially entering ecosystems and affecting wildlife.
- Example: Nanoparticles used in drug delivery systems or medical imaging might accumulate in water supplies if not properly disposed of, posing potential risks to aquatic life.
- Bioaccumulation:
- Given their small size, nanoparticles can travel through biological barriers (such as the blood-brain barrier), but they may also accumulate in unintended areas, potentially causing adverse effects on healthy tissues.
- Example: Nanoparticles intended for targeted drug delivery may inadvertently accumulate in non-target organs or tissues, causing toxicity or unwanted side effects.
Check out this video.
Mitigating Safety Concerns:
- Surface Modifications and Biocompatibility:
- Nanoparticles can be surface-modified to improve biocompatibility and reduce toxicity. Functionalizing nanoparticles with molecules that promote their safe interaction with biological systems can help prevent immune reactions or toxicity.
- Example: Functionalizing nanoparticles with PEG (polyethylene glycol) to prevent immune system recognition, improving their circulation time and reducing potential toxicity.
- Controlled Release and Monitoring:
- Ensuring that nanoparticles release their payload in a controlled manner and only at the target site is crucial for minimizing side effects. Advanced monitoring techniques can help track nanoparticle distribution and clearance in the body, ensuring that they do not accumulate in harmful quantities.
- Example: Nanoparticle-based drug delivery systems can be designed to release drugs only when they encounter the target environment (e.g., an acidic tumor microenvironment), reducing the risk of off-target effects.
- Preclinical and Clinical Safety Testing:
- Extensive preclinical studies (animal testing) and clinical trials are necessary to ensure the safety of nanoparticles before they are used in humans. These tests are critical for identifying potential risks and determining safe dosage levels.
- Example: Clinical trials for nanoparticle-based drugs or medical devices are required to assess their long-term safety and efficacy in human patients.
Real-World Example:
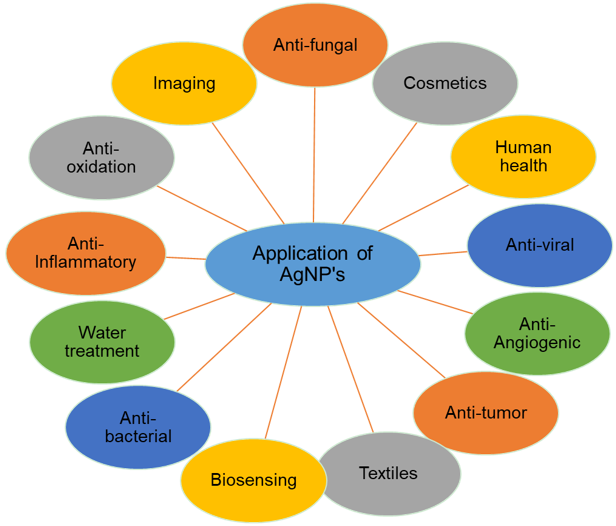
- Silver Nanoparticles in Medicine:
- Silver nanoparticles have been widely used in wound dressings and medical devices due to their antimicrobial properties. However, concerns about their toxicity and potential bioaccumulation have led to increased regulation and safety testing before they are used in medical products.
Read more here.
Case Study:
- Carbon Nanotubes in Medical Devices:
- Carbon nanotubes, used in a variety of medical applications including drug delivery and imaging, have shown promise but also raised concerns about their toxicity. Research has focused on developing safer, biocompatible coatings and understanding their long-term effects in the human body.
Discussion Question:
What do you think is more important: ensuring the rapid development of nanotechnology in medicine, or taking the time to address safety concerns? How should regulatory bodies balance innovation with patient safety?