Challenges in Integrating Nanosensors into Clinical Practice
Objective:
To identify and discuss the challenges associated with integrating nanosensors into clinical neurosurgery practice.
Introduction to Challenges in Clinical Integration:
While nanosensors have tremendous potential to revolutionize neurosurgery, their integration into clinical practice presents several challenges. These challenges range from technological limitations to regulatory hurdles and concerns about patient safety. Understanding and addressing these challenges is crucial for the successful implementation of nanosensors in neurosurgery and other medical fields.
Key Challenges in Integrating Nanosensors into Clinical Practice:
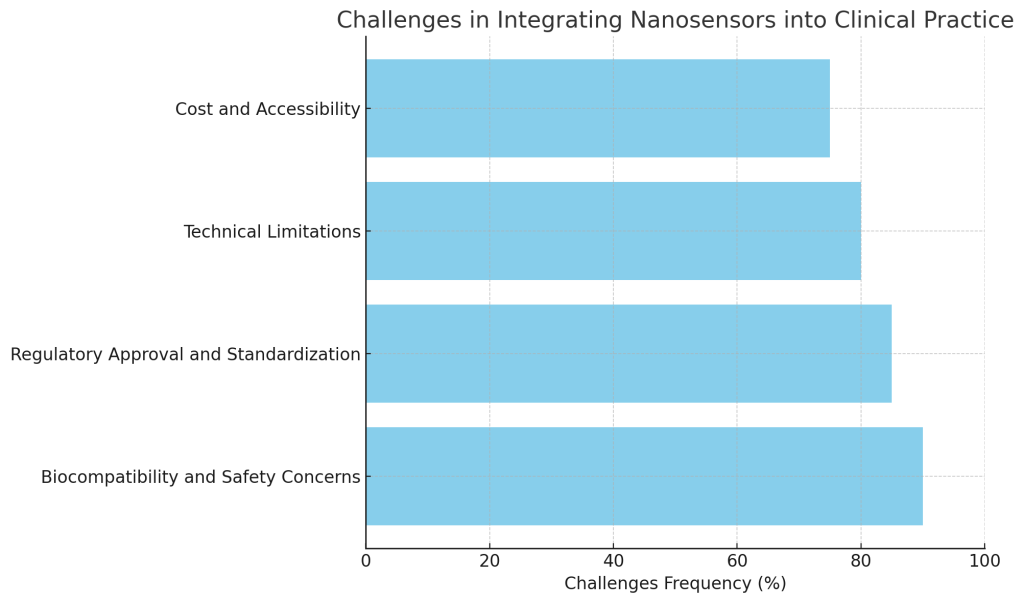
- Biocompatibility and Safety Concerns:
- One of the primary concerns with nanosensors is ensuring their biocompatibility. Since these sensors are designed to interact with human tissues, it is essential that they do not cause adverse immune responses, toxicity, or other complications. Extensive testing and optimization are needed to ensure that nanosensors are safe for long-term use in the body.
- Example: Nanosensors used for brain monitoring need to be biocompatible with neural tissue to avoid inflammation or rejection.
- Regulatory Approval and Standardization:
- Nanosensors are subject to strict regulatory approval processes before they can be used in clinical practice. This process involves demonstrating that the technology is safe, effective, and reliable. Additionally, there is a lack of standardized protocols for the use of nanosensors in clinical settings, which can delay their adoption.
- Example: The approval of nanosensors for use in real-time brain monitoring during surgery requires clinical trials to prove their safety and efficacy, which can take several years.
- Technical Limitations:
- The small size of nanosensors and their integration with other medical devices present technical challenges. These sensors need to provide high sensitivity, reliability, and stability under a range of physiological conditions. Furthermore, their integration with other surgical tools or imaging systems requires seamless technology development.
- Example: Nanosensors designed to monitor brain activity must be able to detect small electrical signals amidst the noise of other biological activity, requiring sophisticated signal processing technology.
- Cost and Accessibility:
- The high cost of developing and manufacturing nanosensors may limit their widespread use, particularly in resource-limited settings. Additionally, training medical staff to use nanosensor-based systems effectively adds another layer of complexity to their clinical integration.
- Example: The initial costs of developing and testing nanosensors may be prohibitive for smaller hospitals or clinics, limiting their availability to larger, more specialized medical centers.
Real-World Example:
- Regulatory Hurdles in Nanosensors for Brain Surgery:
- The approval process for using nanosensors in brain surgery is lengthy and complex. While there is significant promise for these sensors in improving surgical outcomes, navigating the regulatory landscape remains a significant challenge, slowing their adoption in clinical practice.
Case Study:
- Nanomaterial Safety in Clinical Trials:
- In a clinical trial for nanosensor-based brain monitoring, researchers encountered challenges related to the biocompatibility of the sensor materials. While the sensors successfully monitored brain activity, some patients experienced localized inflammation, highlighting the importance of ensuring that materials used in medical devices are completely biocompatible.